DMFC anode electro-catalyst
Cost and durability present two of the most significant challenges affecting the widespread commercialization of fuel cells for diverse applications encompassing stationary power, transportation power, portable power, auxiliary power units, and material handling equipment. Of the different fuel cell types, proton exchange membrane fuel cell (PEMFC), utilizing hydrogen as the fuel operating at low temperature (120 deg. C), has been considered for applications that require faster start-up times, and frequent starts and stops. On the other hand, methanol powered direct methanol fuel cell (DMFC) are well suited for portable power applications in consumer electronic devices wherein the power requirements are low. A significant fraction of the cost arises due to the prohibitively expensive platinum group-metal (PGM) catalysts that are currently used in the anode and cathode to accelerate the electrochemical reactions. Design and synthesis of effective electro-catalysts with improved electrochemical activity, improved durability/stability, reduced precious metal loading to ultra-low level and increased tolerance to air, fuel and system-derived impurities is of paramount importance. In this context, a novel complex sol-gel process (CSG) has been developed by our group to synthesize unsupported nanocrystalline Pt-Ru based binary, ternary and quaternary solid solution having high electrochemically active surface area with excellent electrochemical activity and durable microstructure for the methanol oxidation reaction. Our electro-catalysts thus have the ability to reduce the noble metal loading to an ultra low level.
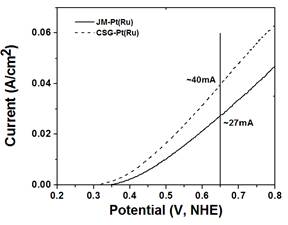
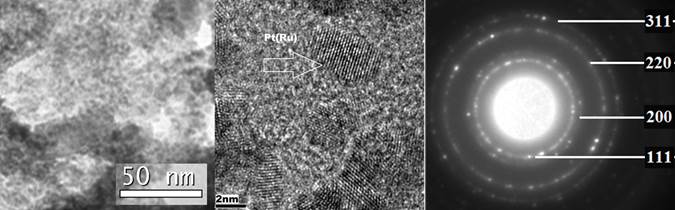
Publication: Y.M. Alyousef, M.K. Datta, K. Kadakia, S.C. Yao, P.N. Kumta, “Sol-gel synthesis of Pt-Ru-Os-Ir based anode electro-catalysts for direct methanol fuel cells”, Journal of Alloys and Compounds, 506 (2010) 698-702.
Affiliated lab members: Karan Kadakia and Moni Kanchan Datta
Hydrogen has been considered as an attractive alternative to fossil fuels particularly over the past decade and is considered by many a harbinger to the hydrogen economy. Furthermore, as the most lightweight fuel it has the potential to provide clean, reliable, and affordable energy supply to meet the growing global energy demand. However a major impediment thwarting commercialization is the inability to economically generate clean and pure hydrogen combined with cost effective storage and distribution strategies. A promising approach to hydrogen production is electricity induced splitting of water using alkaline, neutral or polymer electrolyte membrane (PEM) based water electrolysis. Progress in production of hydrogen fuel by water electrolysis to this date is stymied due to the prohibitive current electrolyzer technology costs limiting attainment of the targeted hydrogen production cost ($3.00/gge delivered) for distributed production systems. The high capital costs of current electrolyzers is due to the deployment of expensive noble metal based electro-catalysts (e.g. IrO2, RuO2, Pt), use of relatively small and comparatively low efficiency systems, combined with the added costs of customized power electronics, and labor intensive fabrication. Rutile type noble metal oxides, IrO2 and RuO2 are well known and are typically accepted as the archetypical standards for oxygen evolution reaction (OER) anode electrode catalysts for alkaline and PEM based water electrolysis. However, the anodic over-potential and the cell resistance in electrolysis contribute to a majority of the losses witnessed in catalytic performance. In addition, IrO2 and RuO2electro-catalysts undergo electrochemical or mechanical degradation under extreme and highly corrosive electrochemical environments prevalent in acid assisted water electrolysis which reduce the performance with time and moreover, diminish the service life of the electrode during OER. We explore different binary and ternary oxide systems along with dopants to identify novel OER electro-catalyst for PEM based water electrolysis.
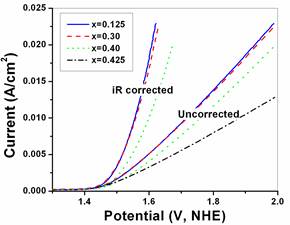
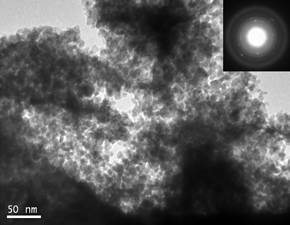
Publications:
1. M.K. Datta, K. Kadakia, O.I. Velikokhatnyi, P. Jampani, S.J. Chung, J.A. Poston, A. Manivannan, P.N. Kumta, “High performance robust F-doped tin oxide oxygen evolution electro-catalysts for PEM based water electrolysis”, Journal of Materials Chemistry A, 1 (2013) 4026-4037.
2. K. Kadakia, M.K. Datta, P. Jampani, S.K. Park, P.N. Kumta, “Novel F-doped IrO2 oxygen evolution electrocatalyst for PEM based water electrolysis”, Journal of Power Sources, 222 (2013) 313-317.
3. O.I. Velikokhatnyi, K. Kadakia, S.K. Park, P.N. Kumta, “Theoretical study of magnesium and zinc tantalates and niobates as prospective catalyst supports for water electrolysis”, Journal of the Electrochemical Society, 159 (2012) F607-F616.
4. K. Kadakia, M.K. Datta, O.I. Velikokhatnyi, P. Jampani, S.K. Park, P. Saha, J.A. Poston, A. Manivannan, P.N. Kumta, “Novel (Ir,Sn,Nb)O2 anode electrocatalysts with reduced noble metal content for PEM based water electrolysis”, International Journal of Hydrogen Energy, 37 (2012) 3001-3013.
Affiliated lab members:
Karan Kadakia, Moni Kanchan Datta, Oleg Velikokhatnyi, Prashanth Jampani Hanumantha and Prasad Patel
Collaborators:
Dr. Ayyakkannu Manivannan (US Department of Energy/NETL, Morgantown)