Bone defects are commonly caused by injury or disease and can lead to a serious health condition that directly impacts the quality of life of sufferers, particularly among the aged. Bone defects up to about 1/3 inch in a large bone can heal by themselves in a healthy patient, but bone defects larger than this, or even smaller in small bones never heal completely. In order to facilitate a more complete and faster bone healing, many surgeons use bone replacement materials or bone graft to fill the defect. Bone graft materials are required to act as a scaffold which assists in integration, healing, strengthening, and improving function. After blood transfusions, bone grafts are the most important set of transplanted materials and these are usually categorized as autografts, allografts and synthetic graft materials.
Unfortunately, the majority of synthetic bone grafts developed to date are non-degradable which lead to insufficient bone formation, poor integration to the surrounding tissue, long term complications and the need for prolonged treatment with antibiotics and immunosuppressive therapies. The poor resorbability may also results in cracks and debris being given off from the synthetic implants which may trigger the onset of infection and lead to further complications. A general lack of porosity and a physiologically relevant pore size distribution in the majority of commercially available synthetic bone graft materials also restricts cellular infiltration, vascularization, and the deposition of extracellular matrix. As a result, this further limits the rate at which native mineralized bone tissues can be regenerated.
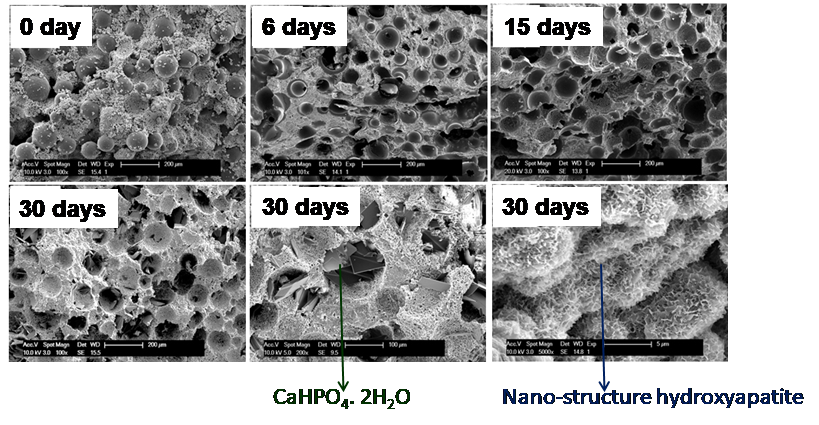
The goal of this project is to engineer a novel injectable, porous, and resorbable calcium phosphate putty (ReCaPP) using FDA approved materials that are clinically easy to adopt and extremely effective at inducing bone regeneration. This unique formulation contains a powder and a liquid which forms putty upon mixing. This injectable putty sets and hardens to a solid form in the defect site even in presence of blood and converts into nanostructured hydroxyapatite similar to natural bone. Bone defect repair frequently involves procedures with limited accessibility and visibility. Injectable and moldable materials however afford the luxury to access and operate within a site with constrained boundaries. In addition to offering minimal invasion, injectability proffers consistency to these materials for application over irregular defect sites, a fact critical for effectiveness in cranio/maxillofacial and periodontal surgeries. The set solid scaffold has sufficient mechanical stability to provide temporary support along with the host tissues throughout regeneration. The presence of interconnected pores and nanostructured hydroxyapatite in this set scaffold allows cellular infiltration and vascularization to occur and thus this scaffold resorbs at a rate similar to the rate at which native mineralized tissues are regenerated. More importantly, this putty supports bone formation as well as bone marrow generation. The putty has demonstrated full bone regeneration in rabbit ulna and calvarial models with critical sized defects (defects too large to heal on their own) by implanting ReCaPP putty, without the addition of any exogenous growth factors. The bone that is formed is identical in nature to natural bone in the body in that it is comprised of about 60% calcium phosphates and 40% bone marrow. It has also been established that bone regeneration and scaffold resorption rates using the developed putty are much higher than those of a commercial gold-standard bone void filler.
Another goal of this project is 3-D printing (also known as additive manufacturing) of scaffolds using the above mentioned patented calcium phosphate putty powder. The greatest successes in additive manufacturing including 3-D printing are currently taking place in the biomedical industry, particularly in the making of implants that take advantage of the technology’s design flexibility to match a patient’s particular needs, such as a customized jaw or hip implants. Printed three dimensional porous scaffolds mimicking the rabbit ulna bone using the ReCaPP powder has been developed. Implantation of these printed scaffolds in rabbits demonstrated the feasibility of this innovative technology for bone regeneration. Moreover, this work also showed that different biological molecules and growth factors can be incorporated into these scaffolds and can be delivered locally to improve the scaffolds integration with the existing bone as well as rapid new bone formation into the defect.
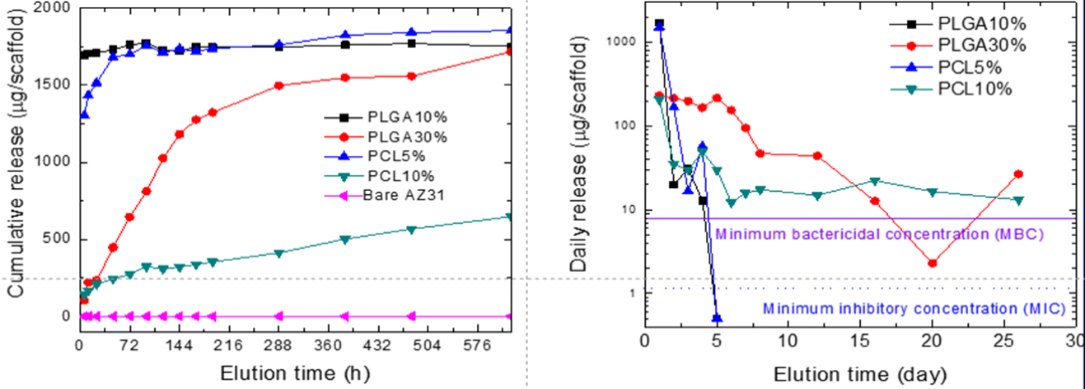
For treating bone related diseases it is highly desirable to provide a sustain release of specific therapeutic agents (e.g. vancomycin, gentamicin) directly at the bone site in need for a prolonged but desired time period. The other advantage of local targeted delivery is that the effective dosage or drug concentration can be achieved at the disease sites, while keeping the systemic drug concentration very low. This helps to avoid any drug related adverse effects to other organs. The current project has successfully developed novel biopolymer-ReCaPP based composite bone putties for long term antibiotic release. Studies showed that these composite putties not only can release antibiotics like vancomycin in a sustained and control manner over time but also form macro-pores due to degradation of the biopolymer which can also assist rapid bone regeneration.
References: BONE SUBSTITUTE COMPOSITIONS, METHODS OF PREPARATIONS AND CLINICAL APPLICATIONS”, P. N. Kumta, C. Sfeir and A. Roy, U.S. Patent No.: US 8,357,364 B2, Date of Patent: Jan. 22, 2013.
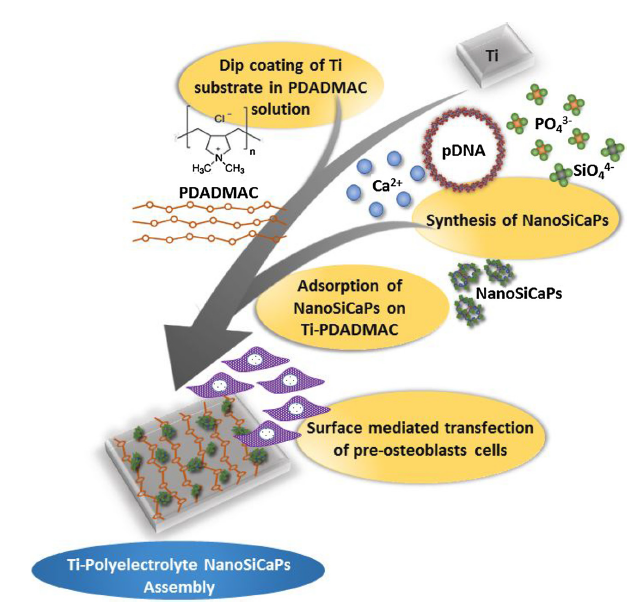
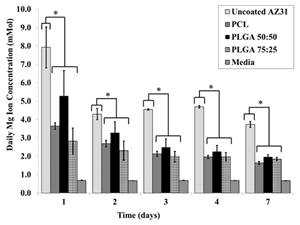
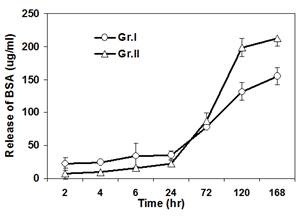
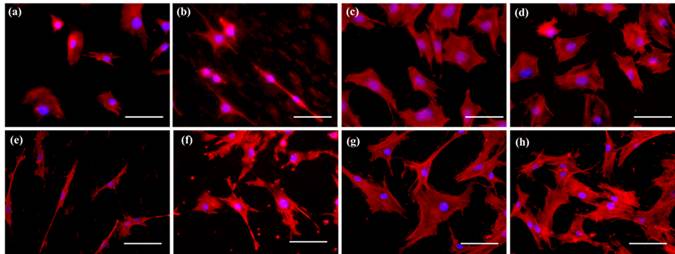
A series of composite degradable, inorganic conversion coatings paired with bioactive polyelectrolyte multilayer (PEM) coatings have been developed for the use on pure magnesium or magnesium-based alloys. Inorganic/PEM coatings may serve many purposes when applied to degradable magnesium implants, including improved biocompatibility and osseointegration, controlled corrosion resistance, and controlled delivery of drugs, growth factors or other biomolecules from the implant surface. The development of such degradable, drug-eluting coatings on magnesium alloy implants could have a significant impact in the field of tissue regeneration including bone and represent convenient strategy to improve the efficacy of stand-alone magnesium alloy implants or other metal based implants. Although previous coating research has attempted to address individual needs of alloy coatings, through ceramic or polymeric coatings, the inorganic/PEM composite coatings pose the potential to address numerous needs with one coating technology. The current work reports the fabrication of multilayered coatings, consisting of natural and synthetic polymers, assembled using layer by layer (LbL) technique under physiological conditions following pretreatment of alloy substrates under alkaline and/or fluorinating conditions. PEM coating systems studied have covered a wide array of natural and synthetic polymers, including the use of alginate (ALG) coupled with poly-L-lysine (PLL) and poly (lactic-co-glycolic acid) (PLGA) or polycaprolactone (PCL) coupled with poly(allylamine hydrochloride) (PAH). Preliminary results collectively show the potential use of LbL coatings on magnesium based degradable scaffolds to improve their surface bioactivity. Release of a model protein, Bovine Serum Albumin, from some of these LbL coated magnesium alloys show control release of the protein with time and hence these novel coating systems can act like smart degradable delivery vehicles for inorganic and organic molecules.
Publications:
*Singh SS, Roy A, Lee B, Ohodnicki J, Loghmanian A, Banerjee I, Kumta PN, “An Evaluation of Strontium Doped Calcium Phosphate Coatings on AZ31”, 2013, 2013, Under Revision
*Nicole Ostrowski, Boeun Lee, Nathan Enick, Benjamin Carlson, Sangeetha Kunjukunju, Abhijit Roy, Prashant N. Kumta. "Corrosion Protection and Improved Cytocompatibility of Biodegradable Polymeric Layer-by-Layer Coatings on AZ31 Magnesium Alloys" Acta Biomaterialia, In Press
*Sangeetha Kunjukunju, Abhijit Roy, Madhumati Ramanathan, Boeun Lee, Joe E. Candiello, Prashant N. Kumta. "Layer by Layer approach to natural polymer derived bioactive coatings on magnesium alloys" Acta Biomaterialia, In Press
*Ostrowski, N., Lee, B., Roy, A., Ramanathan, M., Kumta, PN. (2012) “Biodegradable poly(lactide-co-glycolide) coatings on magnesium alloys for orthopedic applications.” Journal of Material Science: Materials in Medicine 24 (1) 85-96
*Sangheeta K, Roy A, Singh SS, “Novel Alginate Based Coatings on Mg Alloys”, Materials Science & Engineering B 176 (2011) 1703-1710
*Roy A, Singh SS, Lee B, Kumta PN, “Novel Sol-gel Derived Calcium Phosphate Coatings on Mg4Y Alloys”, Material Science & Engineering B 176 (2011) 1679-1689
*Singh SS, Roy, A, Lee B, Kumta PN, “Aqueous Deposition of Calcium Phosphate and Silicate Substituted Calcium Phosphate Coatings on Mg Alloys”, Material Science & Engineering B 176 (2011) 1695-1702
*Sung Jae Chung, Abhijit Roy, Daeho Hong, John P. Leonard, Prashant N. Kumta, Microstructure of Mg–Zn–Ca thin film derived by pulsed laser deposition. Mater. Sci. Eng. B 176 (2011) 1690-1694
* Sung Jae Chung, Abhijit Roy, Daeho Hong, John P. Leonard, Prashant N. Kumta, Microstructure of Mg–Zn–Ca thin film derived by pulsed laser deposition. Mater. Sci. Eng. B 176 (2011) 1690-1694
Patents: "Development of Smart Polyelectrolyte Layer-by-Layer Coating Systems on Mg Alloys for Achieving Tailored Corrosion Properties, Improved Bioactivity and Delivery of Inorganic and Organic Biomolecules" Provisional Patent application filed.
Affiliated Lab Members: Dr. Sangeetha Kunjukunju, Dr. Abhijit Roy, Satish Singh, Sung Jae Chung, Nicole Ostrowski, John Ohodkicki, Sudhanshu Shekhar
Collaborators & Affiliations: NSF Engineering Research Center for Revolutionizing Metallic Biomaterials
The goal of this project is to develop novel biodegradable metals/alloys to be used in orthopedic, cardiovascular, and tracheal medical devices, among many others. These metals are being pursued to avoid complications associated with inert, non-degradable metallic biomaterials such as stainless steel, titanium, and cobalt-chromium alloys. In addition, mechanical properties of biodegradable metals are also superior to resorbable polymers used in medical device implants.
Our research in this area primarily focuses on magnesium and iron based alloy development, with evaluation of their degradation behavior, mechanical properties, and cytocompatibility in suitable physiological conditions. We utilize a variety of processing techniques to tailor the alloys’ properties to suit specifications according to the clinical need. Various processing techniques currently being explored for developing Mg and Fe based alloys include methods such as permanent mold gravity casting, rapid solidification, high energy mechanical alloying, isostatic pressing, and inkjet 3D printing, as well as post-processing routes such as hot extrusion and rolling, and heat treatments. Magnesium and iron based alloys currently developed in our group have demonstrated improved biodegradation behavior compared to their pure metal counterparts, as well as high mechanical properties after post-processing regimes.
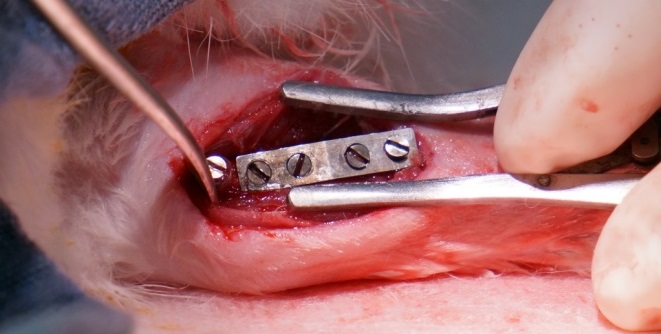
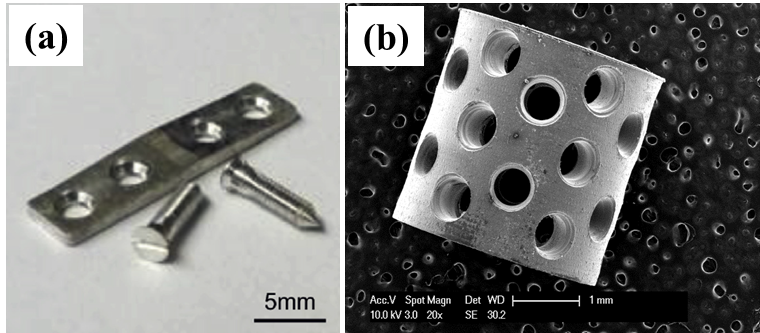
These projects are being conducted as part of the National Science Foundation’s Engineering Research Center for Revolutionizing Metallic Biomaterials (http://erc.ncat.edu) for which collaborations are ongoing with the goal of designing, manufacturing, and testing in vivo medical devices made from the degradable metals. Our alloys have been fabricated into devices such as stents, fixation plates and screws, and tested in animal models.

Publications:
Chou D, Wells D, Hong D, Lee B, Kuhn HA, Kumta PN. Novel processing of Iron-Manganese alloy based biomaterials by inkjet 3D printing. Acta Biomaterialia. Epub ahead of print, 2013.
Datta MK, Chou D, Hong D, Saha P, Chung SJ, Lee B, Sirinterlikci A, Ramanathana M, Roy A, Kumta PN. Structure and Thermal Stability of Biodegradable Mg-Zn-Ca based Amorphous Alloys Synthesized by Mechanical Alloying. Mater. Sci. Eng., B. Proceeding of 2nd Symposium on Biodegradable Metals. 176 (20) 1637-1643, 2011.
Affiliated Lab Members: Dr. Partha Saha, Da-Tren Chou, Dae Ho Hong, Jingyao Wu
Collaborators & Affiliations: NSF Engineering Research Center for Revolutionizing Metallic Biomaterials
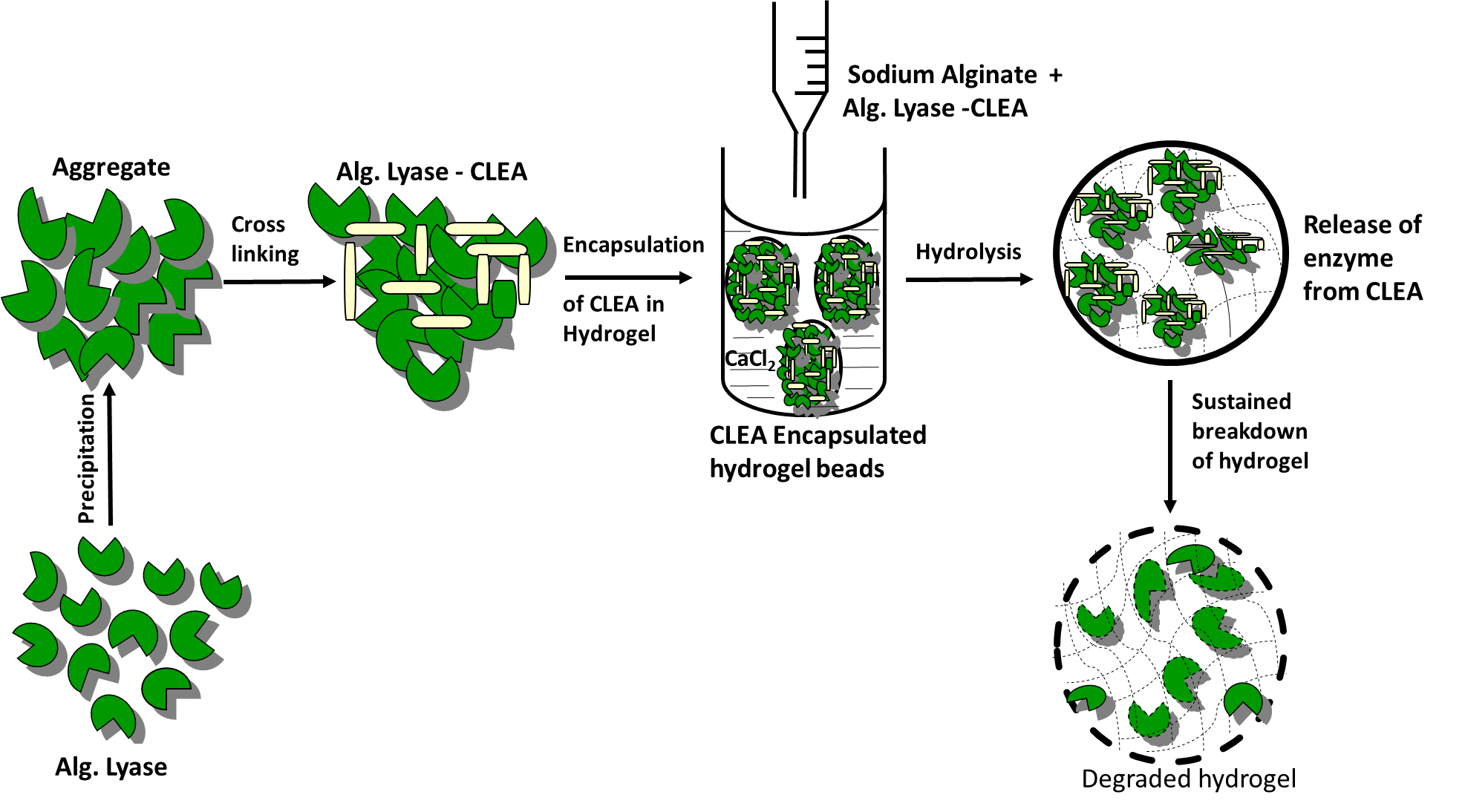
Hydrogels of biopolymers are attractive vehicles for proteins, drugs and cells delivery due to their excellent biocompatibility along with the possibilities of tailoring the release characteristics. However, the slow and uncontrollable degradation of hydrogel are major roadblocks for its successful clinical applications in regenerative medicine. Enzymatic degradation of alginate gels using alginate lyase is fast evolving as an increasingly popular alternative to dissolution of gels, and lyases have been effectively applied in a variety of studies. In this project an enzyme aggregate of alginate lyase (EC 4.2.2.3) from flavobactierium was prepared using ammonium sulfate. The resultant aggregates upon cross-linking with glutaraldehyde produced insoluble and catalytically active cross-linked enzyme aggregate (CLEA) enzyme. The catalytic activity and stability of the cross-linked enzyme aggregate of alginate lyase (CLEA-AL) was studied in the presence of various pH, temperatures and organic solvents. Reusability, storage stability and surface morphology of the CLEA-AL were also studied. By encapsulating CLEA-AL into alginate hydrogel, we demonstrate that alginate hydrogels can be enzymatically degraded in a controlled fashion. The results also showed that degradation of alginate hydrogel with CLEA-AL incorporated beads is slower than native enzyme and therefore, CLEA-AL can be used for controlled degradation and release of various biologics from the degrading gel.
1. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 115, 176–184 (2018)
The broad goal of this project is to design and develop patient specific, customizable as well as complex 3D anatomical shapes from biodegradable ceramics, metals and biodegradable metal-polymer composites with hierarchical porous structures mimicking the macroscopic and internal microstructure of various and specific organs and tissues while also providing the temporary functional mechanical and structural attributes as well as mass transport properties using the binder-jetting 3D printing technique. We have utilized 3-D inject printing, Selective Laser Melting (SLM) and extrusion methods to fabricate various resorbable bone scaffolds. The materials that were used to print these bone scaffolds include iron and magnesium based alloys, ceramics and biopolymers. For examples, we have used calcium phosphate powders in a 3D inject printer to generate rod-shaped scaffolds having interconnected pores on the center and surface of the implants. In-vivo results clearly demonstrated the efficacy of these porous scaffolds for bone-regeneration. Research has been focused on assessing the in vitro degradation, mechanical, and cytocompatibility properties of the 3D-printed samples fabricated using novel patent pending biodegradable Fe based alloy powders. Fe powder was also used to study the mechanical properties of novel engineered biomimetic rotating plywood designs. We applied the rotated plywood structure of bone tissue to generate a biomimetic 3-D printed scaffold with the goal of improving the mechanical performance compared to an orthogonal mesh design commonly used in tissue scaffold applications. Electron beam melting (EBM) of pure Mg powder was also carried out and the results demonstrated the feasibility of this technique to generate 3D constructs of Mg.

- ACTA BIOMATERIALIA 9(10), 8593 (2013).
- ACTA BIOMATERIALIA 45, 375 (2016).
- ACS BIOMATER. SCI. ENG. 3, 648 (2017).
Manifold possibilities exist to release different growth factors, proteins and drugs in a controlled manner from the composite scaffolds so that they can be used not only to engineer rapid bone regeneration but also for effective treatment of different bone related diseases such as osteomyelitis. We have developed various composites cements consisting of bone cements and degradable bio-polymers microspheres and micro-filaments. We have demonstrated that the inclusion of various polymers results in a highly inter-connected porous network. This porous microstructure of the scaffold has been found to be instrumental in facilitating cell infiltration and bone formation. In addition, the in-vitro release of the model agent, vancomycin, from the composite cements suggests that drug association with composite scaffolds can be tuned to control the release kinetics. Further, we have shown the release lasting for longer than 10 weeks from the composite cements in which vancomycin is encapsulated in PLGA microspheres. We have also demonstrated that these porous bone scaffolds can be re-infiltrated with smaller, BMP-2-releasing alginate microspheres and coated with PDGF-releasing alginate hydrogels to deliver a programmed schedule of these growth factors to cell cultures. The chosen delivery schedule of PDGF and BMP-2 has shown to be effective in stimulating cell infiltration of the scaffold, generating angiogenic tubule network formation, and promoting ALP expression, indicating the potential for this hybrid scaffold system to supply the cues to orchestrate vascularized bone regeneration.
1. TISSUE ENGINEERING: Part A, Volume 23, Numbers 23 and 24, 1382 (2017).
2. MATERIALS SCIENCE AND ENGINEERING C 59, 92–101(2016).
Among three major classes of biomaterials: metals, ceramics, and polymers, the metals have gained popularity for load-bearing applications due to their high stiffness and mechanical strength, compared to polymers and ceramic materials. Thus, metallic implants based on Ti (Ti6Al4V), Co-Cr alloys, and stainless steel (SS316L) have been used for more than a decade by clinicians for orthopedic reconstruction. However, a strong mismatch between the elastic moduli and tensile strengths of these metals and natural bone causes stress shielding effects resulting in the weakening and consequent fracture of the surrounding bone. In addition, the long-term use of current inert metal implants often requires invasive secondary surgery to prevent the side effects from wear debris causing metallosis and other related foreign body response. Adult patients are likely to undergo secondary removal surgery only in the case of any eventual complication. On the other hand, pediatric patients are highly likely to require secondary surgeries to remove the implants interrupting bone growth. Therefore, the development of biodegradable metal implants to overcome the limitations of current clinical solutions is of high interest in the field of orthopedics and regenerative medicine. Biodegradable Mg alloy based implant devices will resorb in a controlled fashion thus overcoming the limitations of current metallic implants while keeping the advantage of higher stiffness and mechanical strength. Our group over the past 10 years is working to develop various Mg based alloys with improved bio-corrosion resistance and bio-mechanical properties. In addition, various fixation devices have been developed and tested for their local and systemic toxicities.
- ACTA BIOMATERIALIA, 9(10): p. 8518-8533 (2013).
- ACTA BIOMATERIALIA, 9(10): p. 8534-8547 (2013).
- TISSUE ENGINEERING PART A, 21: p. S172-S172 (2015).
- MATERIALS SCIENCE & ENGINEERING C, 57: p. 294-303 (2015).
- EUROPEAN CELLS AND MATERIALS, Vol. 32 Suppl. 6, page 50, (2016).
- EUROPEAN CELLS AND MATERIALS, Vol. 32 Suppl. 6, page 13, (2016).
Rapid corrosion of magnesium and its alloys in chloride containing solutions, human body fluid, or blood plasma resulting in the uncontrolled release of hydrogen gas have limited their use in clinical applications. Therefore, the development of biocompatible inorganic and/or bio-functionalized coatings that can delay or control the corrosion rate and consequently, the hydrogen gas evolution as well as promote rapid bone regeneration is of immense importance and play a very critical role for the success of Mg scaffolds as an implant. Over the past 10 years our group has successfully developed and optimized stable organic and inorganic coatings on various Mg based alloys to decrease the alloys’ corrosion rate and hydrogen evolution as well as increase the alloys’ surface bioactivity, thus increasing the clinical relevance of Mg based alloys. The coating techniques that we developed are sol-gel, immersion, phosphating bath, layer-by-layer, polymeric dip coatings, MAO, electrophoretic deposition (EPD), fluoride conversion coating, and sodium hydroxide conversion coatings. The in-vivo results have demonstrated excellent biocompatibility of the coatings and have led to delayed degradation of these coated alloys. Furthermore, we have explored the possibility of release of antimicrobial drugs such as vancomycin and model protein bovine serum albumin (BSA) from the inorganic-organic composite coated surfaces of magnesium substrates.
- MATERIALS SCIENCE AND ENGINEERING B-ADVANCED FUNCTIONAL SOLID-STATE MATERIALS, 176(20): p. 1679-1689 (2011).
- MATERIALS SCIENCE & ENGINEERING C-MATERIALS FOR BIOLOGICAL APPLICATIONS, 40: p. 357-365 (2014).
- MATERIALS SCIENCE AND ENGINEERING B-ADVANCED FUNCTIONAL SOLID-STATE MATERIALS, 176(20): p. 1695-1702 (2011).
- ACTA BIOMATERIALIA, 9(10): p. 8690-8703 (2013).
- ACTA BIOMATERIALIA, 9(10): p. 8704-8713 (2013).
- JOURNAL OF MATERIALS SCIENCE-MATERIALS IN MEDICINE, 24(1): p. 85-96 (2013).
- EUROPEAN CELLS AND MATERIALS, Vol. 32 Suppl. 6, page 10, (2016).
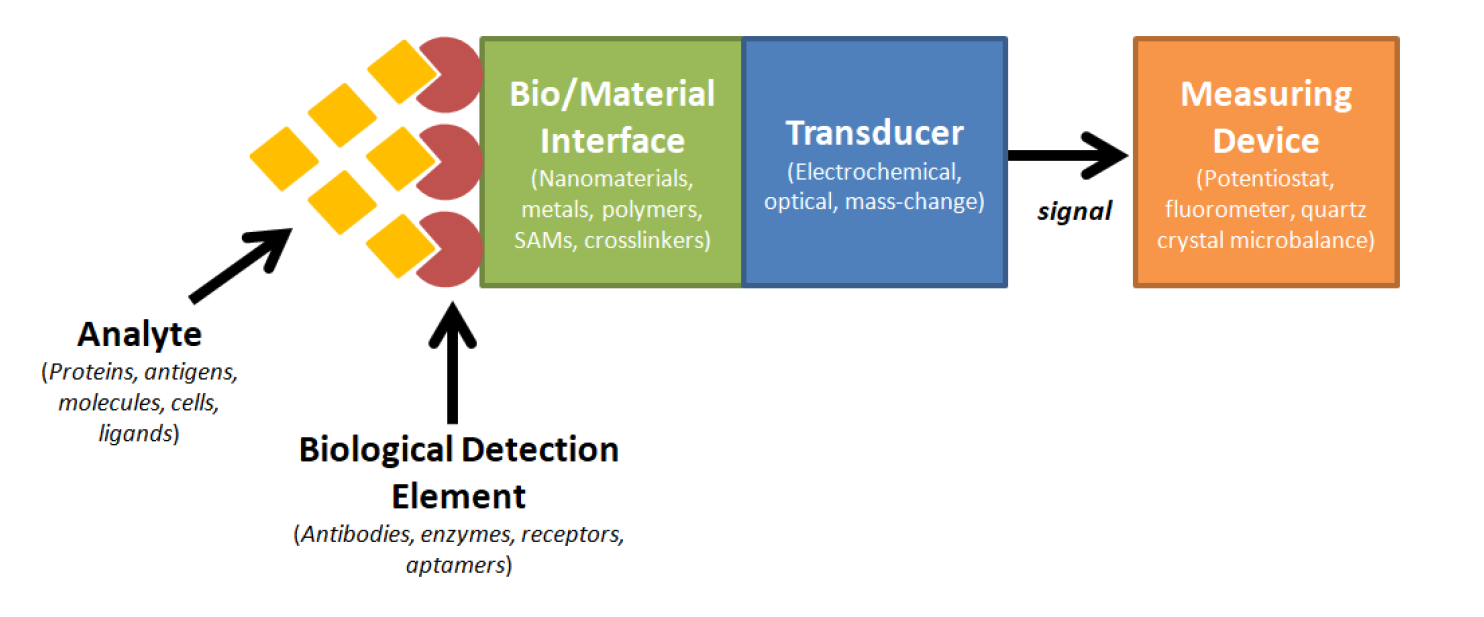
Cardiovascular diseases (CVDs) are the leading national cause of death, impacting nearly 92.1 million Americans and accounting for 801,000 deaths annually. Unfortunately, CVDs are clinically silent until serious complications arise, thus allowing CVDs to go undetected or even be misdiagnosed at earlier stages. In addition, while biomarker testing and other cardiovascular tests can lead to earlier diagnoses, these tests are usually not ordered unless the probability of the patient having a CVD is high due to the expenses, effort, and time required. Therefore, a rapid point-of-care device would be highly useful for screening CVD conditions. This research effort was designed to fabricate a biosensing platform using aptamers and electrochemical impedance spectroscopy to rapidly detect two of the most prominent CVDs, myocardial infarction (MI) and congestive heart failure (CHF). To detect these two diseases, we screened for corresponding biomarkers Troponin T (TnT) and Brain Natriuretic Peptide (BNP). The results demonstrated preliminary efficacy of fabricated biosensor platforms in both serum and whole blood.
- BIOSENSORS AND BIOELECTRONICS, vol. 77, pp. 580-588 (2016).
- ACS BIOMATERIALS SCIENCE AND ENGINEERING, vol. 2, pp. 712-721 (2016).
Bone defect repair frequently involves procedures with limited accessibility and visibility. Injectable and moldable materials however, afford the luxury to access and operate within a site with constrained boundaries. In addition to offering minimal invasion, injectability proffers consistency to these materials for application over irregular defect sites, a fact critical for achieving effectiveness in cranio-maxillofacial and periodontal surgeries. Our research focuses on developing various calcium and magnesium phosphate based injectable bone cements and test their in-vitro and in-vivo bone regeneration efficacies. We have engineered a novel injectable, porous, and resorbable calcium phosphate putty using FDA approved materials that are clinically easy to adopt and extremely effective in inducing bone regeneration in ectopic as well as bone sites (U.S. Patent No.: US 8,357,364 B2, Date of Patent: Jan. 22, 2013). This unique formulation contains a powder and a liquid which forms a bio-reactive bioresorbable putty upon mixing. This injectable putty sets and hardens to a solid form in the defect site even in presence of blood and converts into nanostructured hydroxyapatite similar to the mineral component of natural bone. The putty while undergoing resorption also enables bone formation without the use of any growth factors or biological agents.
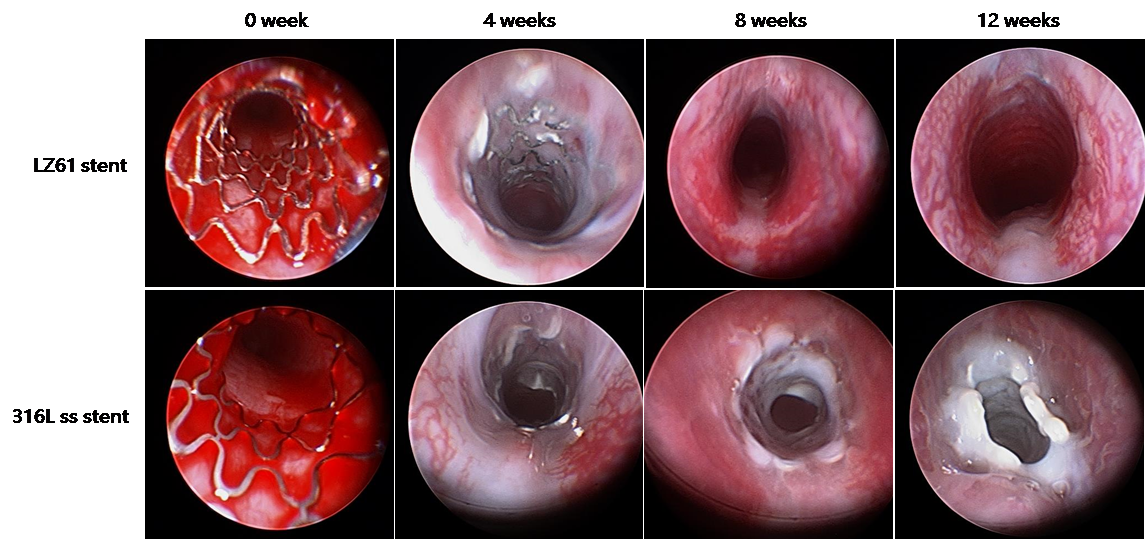
Magnesium alloy based stents have emerged as another biodegradable stent candidate in the past 10 years. Extensive studies that have been conducted report the exploration of different magnesium alloys as novel biodegradable metallic materials for stent application. However, the ductility limitation of magnesium alloy has been a key issue for biodegradable stents applications. Currently, no magnesium alloy based stents have demonstrated similar expansion capability to currently available permanent stents made of bio-inert metallic alloys. The goal of the proposed work was to develop a new magnesium alloy system which addresses the limited ductility of current magnesium systems and to evaluate the feasibility of the proposed magnesium alloy system in the form of prototype tracheal stent devices in clinically relevant animal models. Various biocompatible alloying elements determined by first principles theory were used to synthesize UHD alloys. Tensile test results showed that the Mg-Li alloys displayed lower strength, but higher ductility compared to AZ31 measured by elongation at fracture. Balloon expandable tracheal stents were designed using biodegradable Mg-Li (LZ61) alloys due to its excellent biomechanical and ductility properties compared to commercially available Mg alloy. Further, LZ61 exhibits low in-vivo and in-vitro corrosion rates providing sufficient mechanical support during the entire healing process eliminating pre-mature tracheal collapse, Moreover, Li, and other elements are selected as alloying elements due to their proven safety profiles.
- ACS BIOMATERIALS SCIENCE & ENGINEERING, 2018, 4(3): p. 919-932 (2018).
- ULTRAHIGH DUCTILITY, NOVEL Mg-Li BASED ALLOYS FOR BIOMEDICAL APPLICATIONS, WO2016094510A1, PCT/US2015/064694 (2014).
- JOURNAL OF BIOMEDICAL MATERIALS RESEARCH PART A, 102(3): p. 611-620 (2014).