Research
Ongoing projects at the Translational Imaging Laboratory
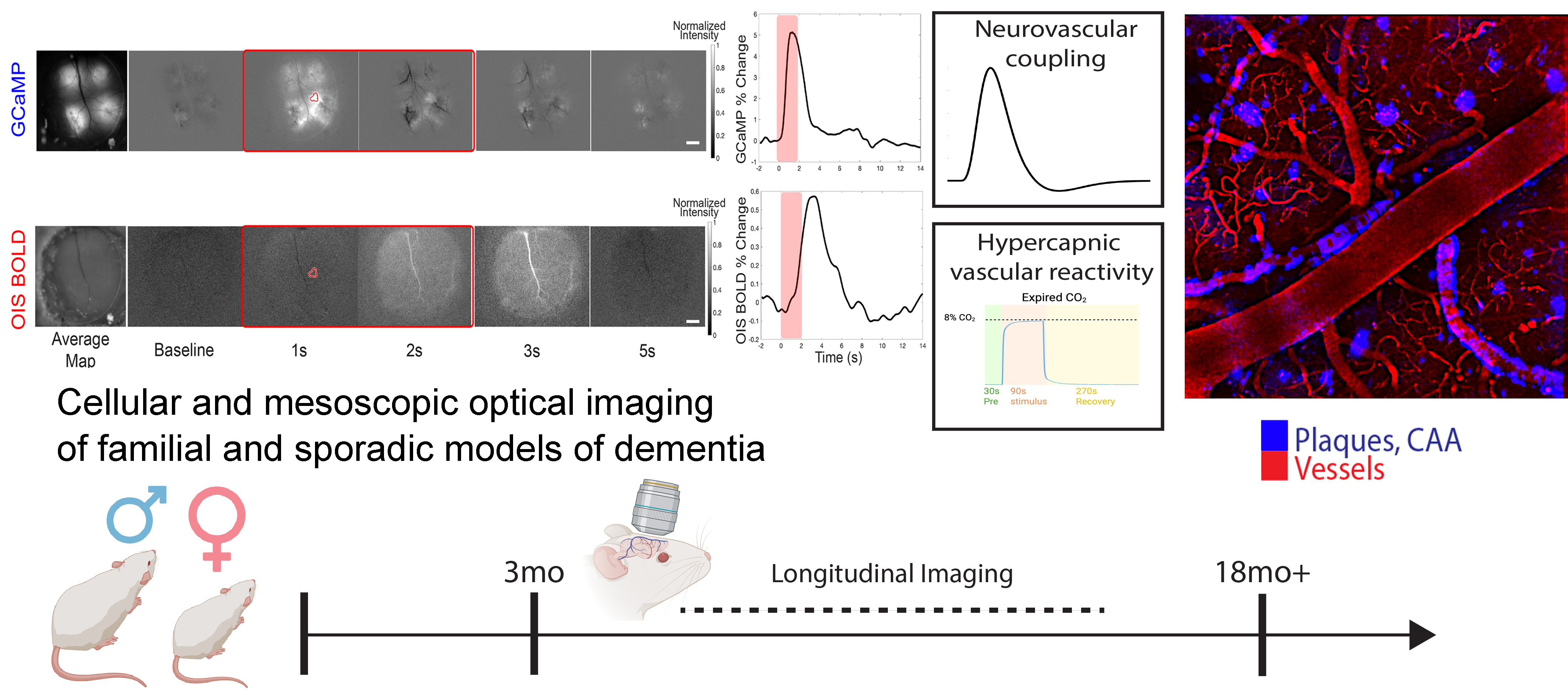
Vascular contributions to Alzheimer’s disease
The broad acknowledgment of vascular contributions to dementia propelled the filed of neurovascular coupling focused on the link between neuronal signaling and cerebral blood flow. This is important because the energetic cost of the mammalian brain is high, but the local energy storage is scarce, and dysfunction of specific cells can have dramatic repercussions on cognition. Vascular dysfunction plays a critical role in the early stages of Alzheimer’s disease (AD), commonly associated with amyloid-β (Aβ) deposition. This vascular dysfunction is particularly relevant in the context of cerebral amyloid angiopathy (CAA), where Aβ accumulates within cerebral vessel walls. Notably, sex differences impact the progression of both AD and cerebrovascular dysfunction, with post-menopausal females displaying increased small vessel disease burden and diminished CO2 reactivity. Moreover, the cerebrovasculature is a target of sex hormones where they exert substantial influence in numerous vascular functions and pathologies across lifespan. Combined, cerebrovascular dysfunction along with Aβ deposition may have differential effects on the sexes. Despite observational studies in humans, preclinical mechanistic and functional research on sex-specific vascular differences in AD has been limited.
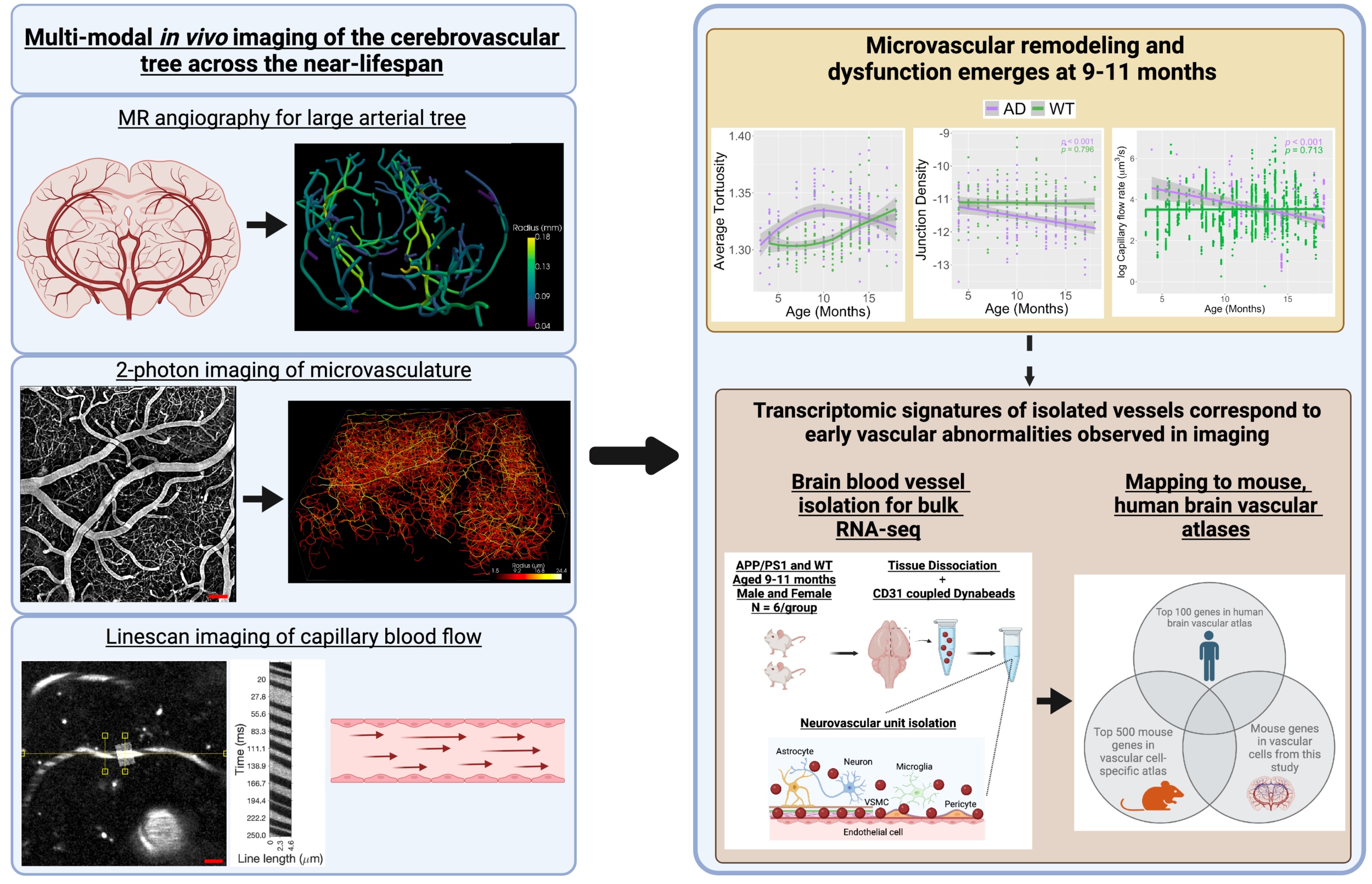
Cross-species trajectories of cerebrovascular remodeling
Growing evidence implicates dysfunctional brain vasculature in the asymptomatic stage of Alzheimer’s disease (AD). Few studies link early neuroimaging observations of vascular abnormalities to transcriptomic signatures from brain vessel cells. Importantly, old age is the biggest risk factor for dementia, yet we lack understanding of how the ageing brain remodels the vascular architecture to sustain energy for neuronal networks. In this research direction, we employ multiscale longitudinal in vivo imaging of the cerebrovascular tree in translational AD models to identify when vascular abnormalities emerge. We have observed decreased capillary blood flow, reduced vascular density and increased small vessel tortuosity. Importantly, using 7T MRI of aging humans, we identified a remarkably similar tortuosity trajectory in the smallest human brain vessels. Bulk RNA-sequencing of isolated brain vessels at this time revealed gene pathways involving neuroinflammation, dysfunctional angiogenesis, and actin-mediated contractility deficits. Mapping our mouse transcriptomic dataset to human vascular atlases identified endothelial and mural cell vulnerabilities with direct translational application. Our integrated framework across scales and species advances cerebrovascular biomarker understanding and informs on potential early-stage vascular therapeutic targets for AD.
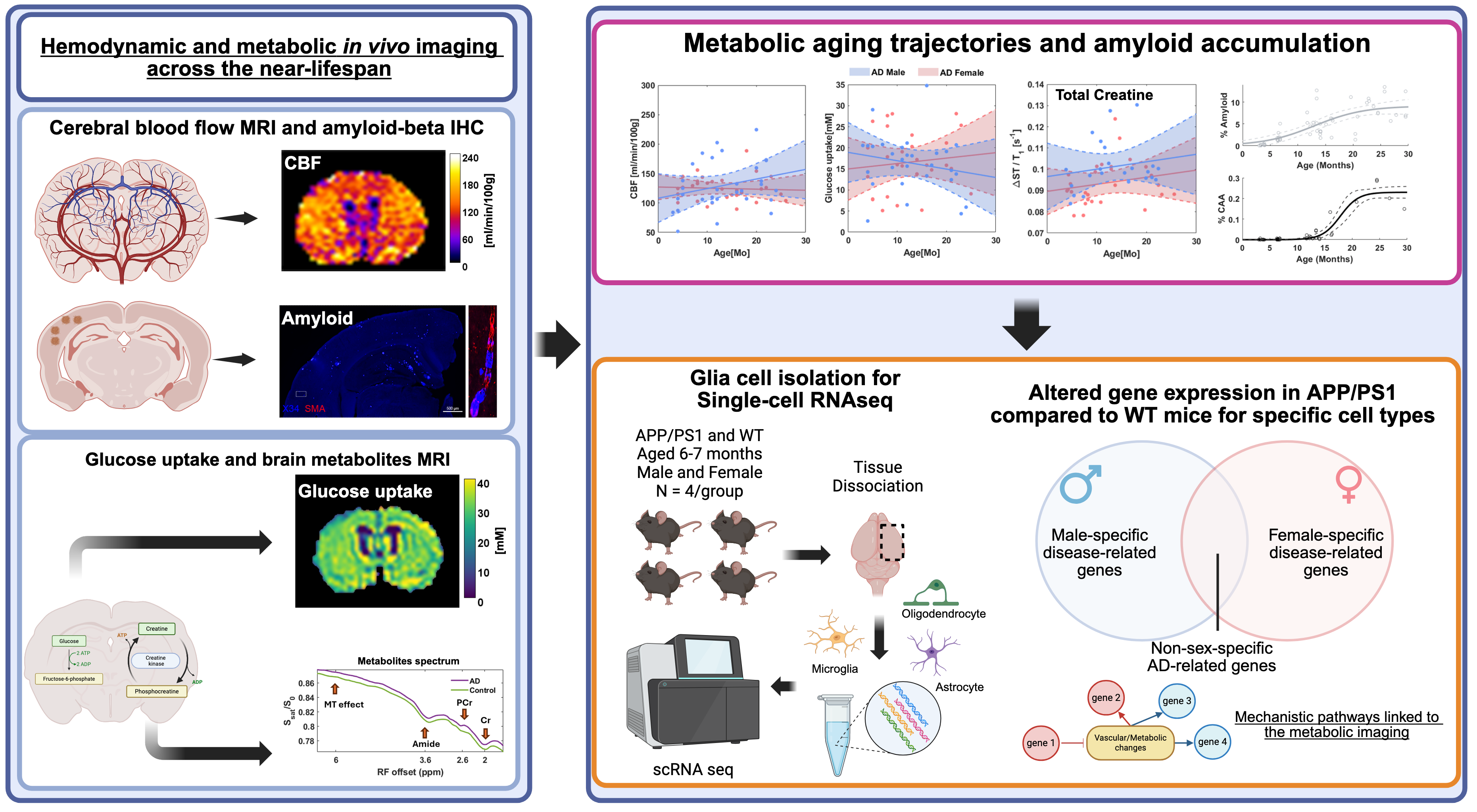
Brain metabolism during aging
Cognition is intricately linked to the metabolic processes of the brain, yet existing brain models often overlook the metabolic costs associated with cognitive function. This oversight is critical, especially in neurodegenerative diseases like Alzheimer's, where metabolic dysfunctions play a significant role in cognitive decline. Despite advancements, research biases towards familial AD models have hindered a comprehensive understanding of metabolic changes in aging and late-onset AD, calling for focused investigations into sporadic late-life AD. This research direction aims to bridge this gap by comprehensively studying neuro-metabolic coupling using state-of-the-art imaging techniques and computational models. We take a multifaceted approach involving in vivo two-photon microscopy, wide-field imaging, and chemical exchange saturation transfer CEST MRI to elucidate the intricate relationship between neuronal activity, functional hyperemia and metabolic processes such as oxidative phosphorylation, glucose, lactate, and creatine dynamics. Through non-invasive brain imaging techniques, we explore the impact of glucose and creatine metabolism on whole-brain functional connectivity. We integrate data from animal models and human cohorts to identify metabolic biomarkers of cognitive decline. This research direction addresses critical gaps in our understanding of neuro-metabolic coupling in aging and late-onset AD, offering insights into metabolic vulnerabilities and potential targets for personalized therapeutic interventions. By integrating data from animal models and human cohorts, we aim to uncover new insights into the metabolic underpinnings of cognitive decline, advance early diagnosis and develop more accurate metabolic biomarkers for AD.
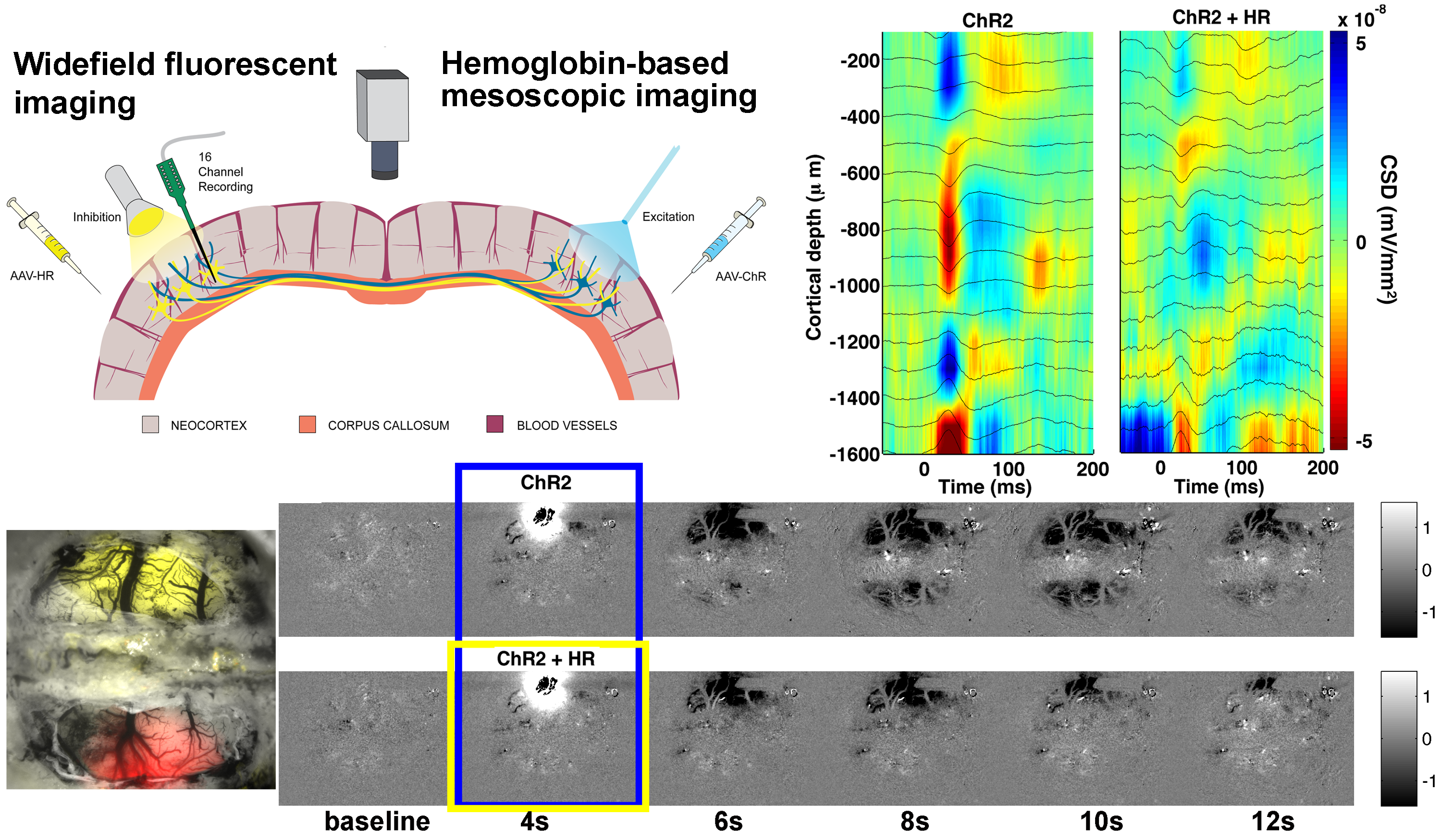
Left-right brain interaction
The interhemispheric circuit connecting the left and the right mammalian brain plays a key role in integration of signals from the left and the right side of the body. The information transfer is carried out by modulation of simultaneous excitation and inhibition. This interhemispheric pathway is crucial for bilateral integration of sensory, motor, and associative functions. Anatomical studies of interhemispheric connectivity implicate large pyramidal neurons in layers II, III and V projecting fibers along the corpus callosum. These long-distance axons terminate on the dendritic spines of both local excitatory and inhibitory postsynaptic cells in layers II, III and V of the contralateral hemisphere. Circuit mapping studies show that transcallosal stimulation elicits postsynaptic excitatory currents that are followed by postsynaptic inhibitory currents and the balance between those seem to vary across studies and experimental paradigms.
Hemodynamic studies of this circuit are inconsistent since little is known about neurovascular coupling of mixed excitatory and inhibitory signals. The goal of this project is to examine interhemispheric neurovascular and neurometabolic coupling in the healthy brain and use this knowledge to discriminate brain dysfunction after unilateral stroke.